IMD International Medical Devices has raised funds
IMD International Medical Devices has completed an IPO on the Euronext Growth Milan market, raising a total of US$6.5 million. The funds will be used to strengthen its competitive positioning in the market and implement a growth strategy, both in Italy and abroad.
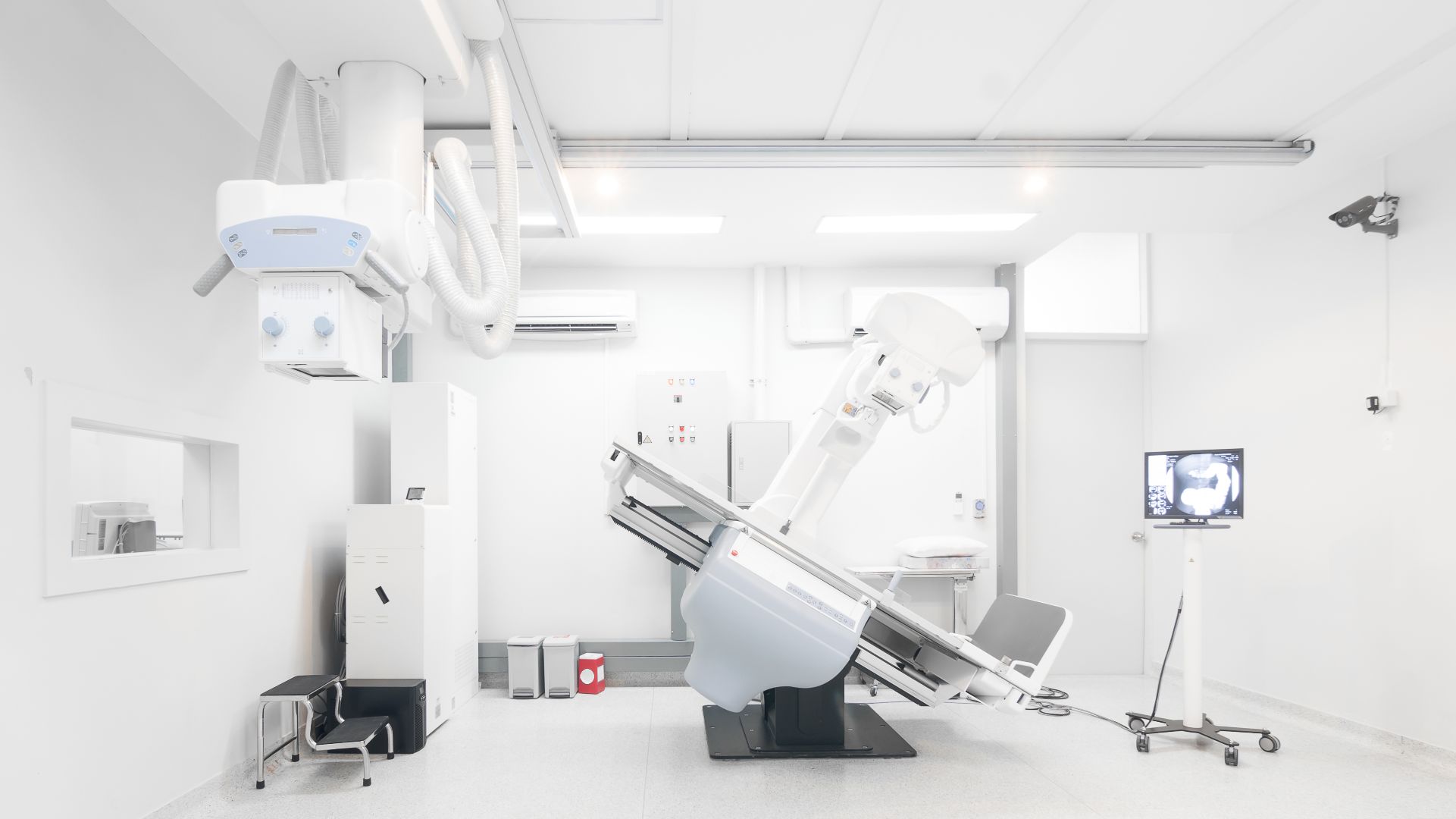
IMD International Medical Devices designs, develops, manufactures and markets diverse X-ray diagnostic systems as well as components for radiology and medical clinics.
Oaklins Italy’s parent company Banca Akros acted as the Euronext Growth advisor, global coordinator and joint bookrunner in this transaction.

Talk to the deal team
Related deals
Backspin has completed a mandatory public tender offer for the shares of Spindox
Backspin S.p.A. has completed a mandatory public tender offer for Spindox S.p.A.
Learn moreRare Patient Voice has been acquired by Konovo
Rare Patient Voice has been acquired by Konovo, a technology-first healthcare intelligence company backed by Fraser Healthcare Partners.
Learn moreLindenhofgruppe has sold its majority stake in LabPoint to Affidea Switzerland
LabPoint Medical Laboratories AG has been acquired by Affidea Switzerland AG. Through the transaction, Lindenhofgruppe AG gains a strong strategic partner to support the further development of LabPoint and will remain a shareholder with a reduced stake, continuing as a key customer of the company. It lays the foundation for LabPoint’s sustainable development under a new anchor shareholder, with the aim of further strengthening and selectively expanding its position in laboratory diagnostics.
Learn more


